
Nitrogen and Phosphorus, the agronomic macronutrients par excellence
I think most people are familiar, in one way or another, with the characteristics of the chemical elements we are going to talk about in this post: nitrogen (N) and phosphorus (P). Nitrogen in its gaseous from (N2) is part of the composition of atmospherica air or we even know it in another of its typical forms, ammonia (NH3), either as a gas or as a liquid solution (in this case as ammonium NH4 ). Phosphorus, on the other hand, is involved in vital functions in living organisms, as well as being one fo the main components of RNA and DNA molecules and is used to store and transport energy in the form of adenosine triphosphate (ATP). Well, today in this post we are going to go deeper into why these two elements are also important for other issues related to human beings and their development, we will explain the importance of nitrogen (N) and (P) as agronomic nutrients and how they are related to the concept of Circular Economy (a concept that has been very topical in recent years). Therefore, from now on, when we talk about nutrients in this post, it will always be focused from an agronomic point of view and nto from a human food point of view. Let´s start!
Both nitrogen (N) and phosphorus (P), together with potassium (K), form the group of agronomic macronutrients, which are the three main macroelements that plants or crops need to incorporate for their growth. Thus, in most cases, the fertilisers that are synthesises, and used nowadays in agriculture have an important composition of these elements (we usually talk about the NPK content in these products).
The first uestion to ask is how are these fertilisers synthesisesd?
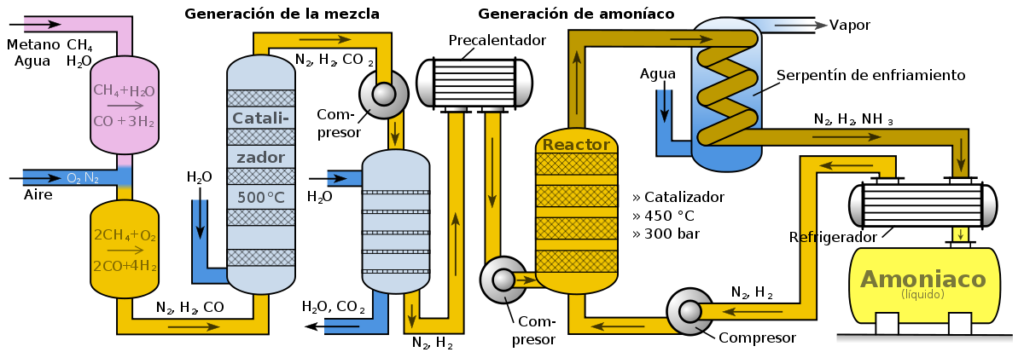
Almost all of the N used in the formulation of fertilisers is obtained from the synthesis of ammonia, the classic procedure for obtaining ammonia being the Haber-Bosch process. Subsequently, the ammonia obtained by the Haber-Bosch process is oxidised to give rise to nitric acid (HNO3), from which the main mineral fertilisers can be obtained, synthesised from ammonium nitrate [(NH4)NO3]. The other main source of N for fertilisers synthesis is urea [(NH2)2CO]. As far as phosphorus is concerned, the main raw materials for its use in fertilisers is apatite, which is a set of minerals obtained through the extraction of the mineral phosphate rock. Therefore, the first thing we can realise is that, in both cases, the origin of N and O for obtaining traditional fertilisers is a non-renewable origin.
In addition to this, there is another factor of great importance, namely the increase in the world´s population. According to United Nations (UN) forecasts, the world population will reach 8.6 billion in 2030 and 9.8 billion in 2050. It is clear that these facts will lead to a significant increase in pressure from the food industry, which will be forced to increase its production, leading to more intensive agricultural practices and therefore an increase in land use and consumption of water, energy and traditional non-renewable fertilisers. Another worrying fact abut this scenario is that the countries of the European Union (EU) are tremendously dependent on imports of these compounds that act as raw materials fot fertilisers. To give you an idea, the EU imports around 30% of the N, more than 60% of the P and 70% of the K of the ttal nutrients consumed as fertilisers products in its countries. This issue is even more dramatic in the case of P, as five countries worldwide hold 90% of the world´s reserves (China, Morocco, South Africa, the United States and the Jordan region). This has led the EU to classify P as a Critical Raw Material (COM(2017)490), as it is crucial for the EU´s own growth, competitiveness and especially for a sustainable food industry.
Against this backdrop, it is clear that the search for and introduction of alternative and renewable soruces of N and P, as well as novel technologies for the production of sustainable fertiliser products, is necessary.
And this is where the Circular Economy comes into play, on the one hand, and the concept of nutrient recovery on the ohter. Nutrient recovery is one of the main lines of research that we have been developing in recent years within the Circular Economy area of the CARTIF Technology Centre. Nutrient recovery consists of the development of methodologies, techniques and technologies that make it possible to obtain the N and P they contain from sustainable raw materials and that these elements are in a convenient and effective form for their subsequent use in the synthesis of bioproducts or sustainable fertilisers that can replace traditional mineral fertilisers or, failing that, increase the renewable component in the synthesis of the latter. It is important to highlight that, although nutrient recovery is mainly focused on the recvery of N and P, the recovery of ther agronomic macro and micronutrients, such as K, magnesium (Mg), calcium (Ca) ,etc., can also be achieved.
So what raw materials or sources of renewable origin can we use for nutrient recovery?
Nutrient recovery mainly focuses on two groups: agricultural and livestock waste and wastewater. By agricultural and livestock waste we mean any waste generated directly by agricultural or livestock activity (manure, slurry,etc.), as well as wastewater (both urban and industrial). In addition, and related to the above, biological waste or by-products obtained in the treatment of such waste could also be used in the recovery of nutrients (a clear example would be the digestate obtained from the treatment of such waste by anaerobic management of the sludge obtained in wastewater ttreatment processes in Wastewater Treatment Plants (WWTPs),etc.).

An important aspect to highlight is that nutrient recovery technologies depend to a large extent on the characteristics of the raw material we use to recvoer N and P and how this raw material is presented (in solid or liquid state). Thus, the simplest methods of nutrient recovery are the direct use as fertilisers of solid wastes or by-products such as activated sludge or manures and digestate or the composting of these. However, logistical aspects (cost of transport and management of the waste, which often contains high moisture) can make the process unfeasible. At the same time, direct application of the waste does not provide effective fertilisation and can lead to overfertilisation phenomena that can trigger eutrophication phenomena (due to the accumulation of N and P present in the soil that has not been assimilated by the crop and can subsequently be washed away by rain or run off and finally deposited in aquifers and bodies of water), with the consequent environmental damage. In additionm the residues may contain significant cmounts of potentially hazardous contaminants, which need to be removed prior to their use as fertilisers. For this reason, waste treatment technologies for N and O recovery are becoming increasingly popular. There are different technologies to recover N and P from liquid wastes, such as biological treatments, stripping, crystallisation, membrane filtration, thermochemical methods (pyrolysis and gasification) or physical treatments (concentration, drying,etc.).
But as all this is best understood with an example, we will try to explain one of the processes in which we have investigated in CARTIF some of our projects.
It is the recovery of nutrients from crystallisation. Crystallisation is a separation operation frequently used in Chemical Engineering, thanks to which purification of fluids is produced through the formation of solids, taking into account the solubility of the products that are of interest for their separation. Thus, crystallisation can be used to recover N and P from wastewater or liquid agricultural and livestock waste (liquid phase of digestate and manuse or slurry) in the form of struvite.
But, wait a minute, let´s take it one step at a time, what is struvite?

Struvite is a salt (mineral orthiphosphate) containing magnesium, ammonium and phosphate in equal molar concentrations, specifically, struvite in the form of magnesium ammonium phosphate hexahydrate has the following molecular formula MgNH4PO4-6H20. Struvite crystallisation occurs easily when the ideal conditions are met (presence of a significant Mg, N and P contract, pH,etc.). In fact, struvite gained public attention in the 1960s as a result of the clogging of pipes in WWTPs, in which it crystallised spontaneously.
And now, we may think, okay, we know what struvite is, but how is it obtained?
The waste to be used as a raw material to extract N and P (normally wastewater or digestate obtained from the anaerobic digestion of waste such as pig slurry) is simply introduced into a crystallisation reactor and a certain amount of magnesium is added (normally in the form of MgCI2 or MgO) and depending on the pH of the reaction mixture, a base (NaOH) can be added to raise the pH (8-9). Once all the components are in the reactor, agitation (either mechanical or by aeration) is applied.
The Mg comes into contact with the N and P of the raw material and little by little the struvite crystals grow, according to the following chemical reaction:
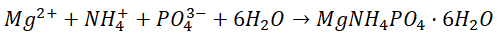
After approximately one hour of reaction, most of the P contained in the raw material (and an equivalent amount of N and Mg) is recovered in the form of a whitish solid crystal, struvite. This solid has very good properties for use as a fertiliser, as struvite has a high concentration of P and, due to its physical characteristics (low solubility), the product can be used as a slow-release fertiliser, i.e., unlike traditional fertilisers, struvite releases nutrients according to the needs of the plant and its stage of growth, making it a more effective fertiliser and avoiding eutrophication and similar phenomena.
There are several struvite crystallisation technologies on the market (with different configurations, reactor types, morphologies,etc.), most of them focused on obtaining struvite from wastewater, however, in CARTIF we have developed a 50L pilot crystallisation reactor, trying to solve the technical impediments presented by other technologies. This crystalliser consists of a fluidised bed reactor,i.e. the agitation of the reaction mixture is achieved in suspension, facilitation their interaction and favouring the formation and growth of the crystals. The struvite crystalliation technology we have developed has been tested in several projects in which we have participated, such as Nutri2Cycle and Nutriman (both European projects of the Horizon2020 programme), with very promising results in the crystallisation process (achieving P recovery yields of voer 90%) and good agronomic performance of the final product obtained (struvite).
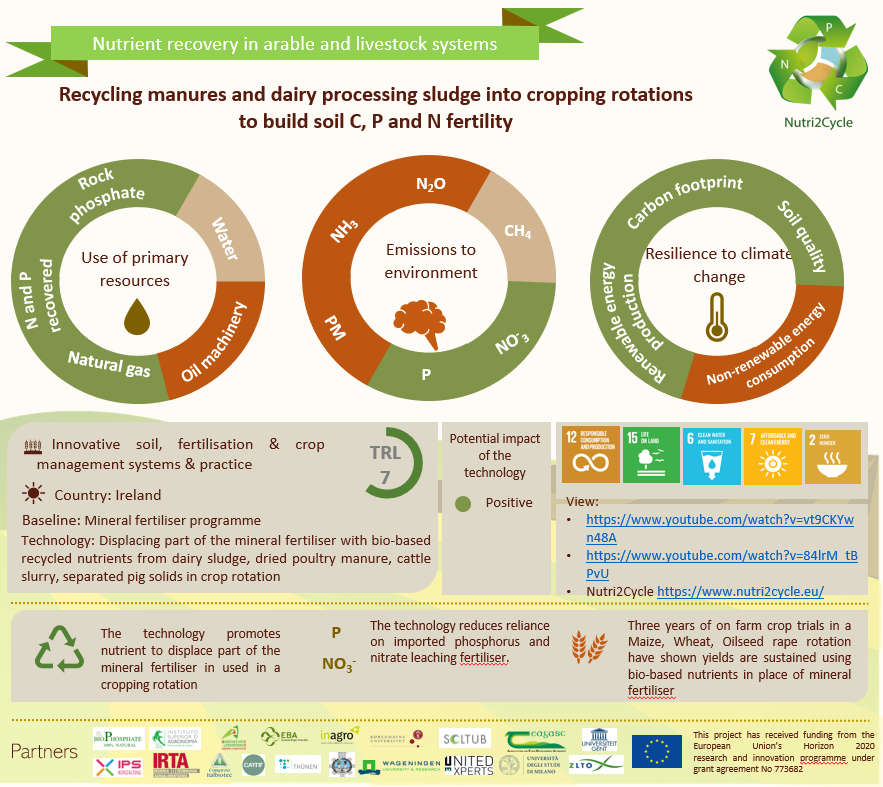
Therefore, as we have seen, thanks to nutrient recovery technologies we have developed sustainable processes in which we recover waste (wastewater, digestate,etc.) following the principles of the Circular Economy and we obtain a biofertiliser of renewable origin with good agronomic performance and with characteristics that are not present in traditional fertilisers of non-renewable mineral origin (slow release). Therefore, struvite would be a good candidate to replace or reduce the use of non-renewable fertilisers.
Currently in the Circular Economy Area of CARTIF, we continue working on the development of this line of research and we are currently coordinating the WalNUT project (another European project of the Horizon2020 programme), in which together with 13 other partners from several European countries we are developing new technologies for the recovery of nutrients from wastewater (both urban and industrial). Specifically, in the case of CARTIF, we are working on a technology for N and P recovery through the cultivation of microalgae and on another technology in which nutrients are recovered through bioelectrochemical processes, i.e. Microbial Fuel Cells (MFCs).
But if you like, we´ll leave that topic for another future blog post ?
See you next time!